Have a JATENZO question?
Connect with an account manager for support, or request medical information about JATENZO clinical trials and safety.

LIVER FUNCTION
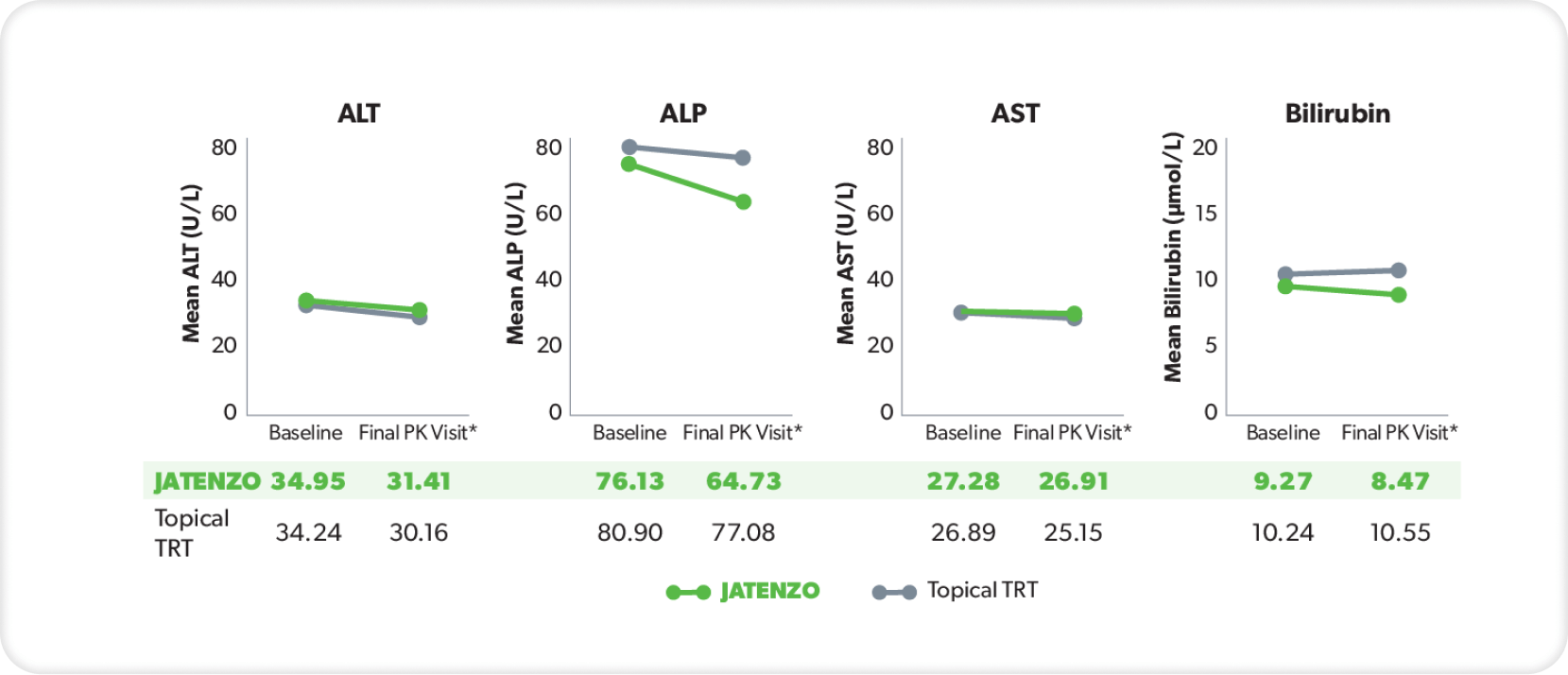
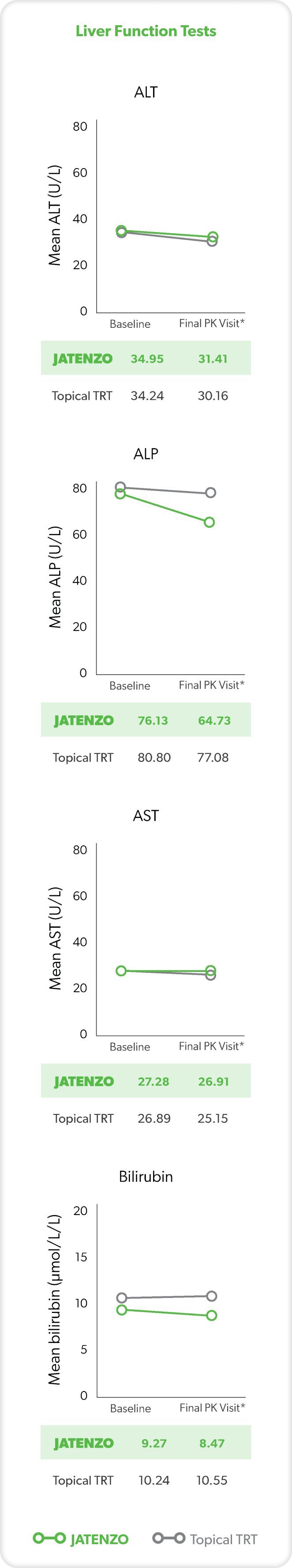
These analyses are from the inTUne clinical study but were not used in formal safety assessments.2
Additional details:
In three patients treated with JATENZO who had clinically significant AST or ALT values, none of the elevations observed were more than twice the ULN for either parameter.3
*Final visit was defined as either Visit 7 or date of last data collection if terminated early.2
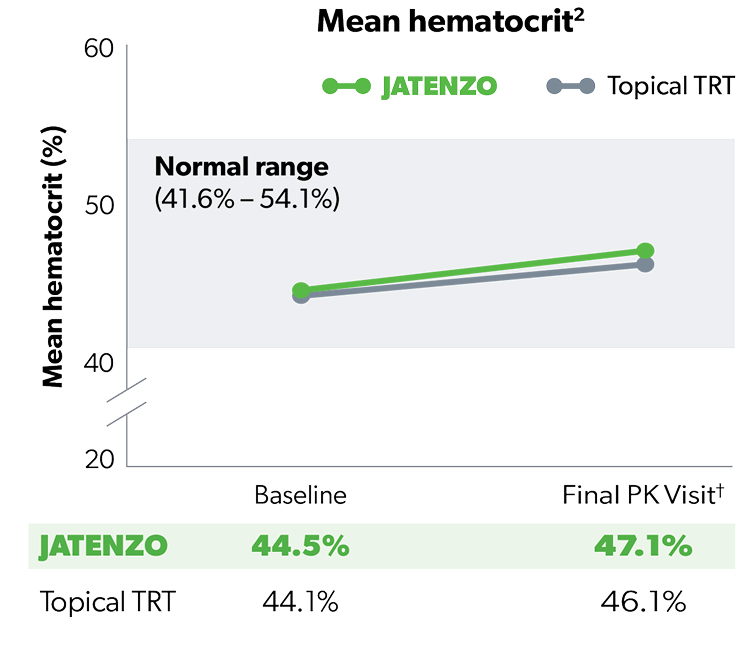
HEMATOCRIT
97% of JATENZO Patients’ Hematocrit Levels Stayed Within Normal Range3
Increases in hematocrit did not lead to any premature discontinuation3
Evaluate hematocrit approximately every 3 months while the patient is on JATENZO.1 All testosterone therapies, including JATENZO, require ongoing monitoring of hematocrit levels.1,4
†Increases in hematocrit were reported in 8 of the 166 (4.8%) patients, which occurred in the second half of the study.1
SAFETY
Most Common Adverse Reactions Reported in >2% of Study Population1
Three patients (1.8% of 166) had adverse reactions that led to premature discontinuation from the study, including rash (n=1) and headache (n=2).1
Adverse reactions reported in >2% of patients in all Phase 2 and Phase 3 JATENZO trials combined (N=569): Polycythemia, diarrhea, dyspepsia, eructation, peripheral edema, nausea, increased hematocrit, headache, prostatomegaly, and hypertension.1
Jatenzo increased systolic/diastolic BP by an average of 4.9/2.5 mmHg from baseline measured by ABPM: Instruct patients about the importance of monitoring blood pressure periodically while on JATENZO.1
| OVERALL (N=166) |
|
|---|---|
| Headache | 8 (4.8%) |
| Hematocrit increased | 8 (4.8%) |
| Hypertension | 6 (3.6%) |
| High-density lipoprotein decreased | 5 (3.0%) |
| Nausea | 4 (2.4%) |
ABPM=ambulatory blood pressure monitoring; ALP=alkaline phosphatase; ALT=alanine aminotransferase; AST=aspartate aminotransferase; BP=blood pressure; PK=pharmacokinetics; TRT=testosterone replacement therapy; ULN=upper limit of normal
Connect with an account manager for support, or request medical information about JATENZO clinical trials and safety.
JATENZO® (testosterone undecanoate) capsules, CIII, is an androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone:
Safety and efficacy of JATENZO in men with “age-related hypogonadism” and in males less than 18 years old have not been established.
JATENZO is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, in women who are pregnant, or in men with a known hypersensitivity to JATENZO or its ingredients.
Increase in hematocrit and polycythemia. High red blood cell counts increase the risk of clots, strokes, and heart attacks.
Venous thromboembolic events (VTE). Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients using testosterone replacement products like JATENZO.
Benign prostatic hyperplasia (BPH). Patients may see worsening signs and symptoms of BPH.
Prostate cancer. Patients treated with androgens may be at increased risk for prostate cancer.
Blood pressure increases. JATENZO can increase blood pressure. Monitor blood pressure periodically in men using JATENZO. Not recommended for use with uncontrolled hypertension.
Abuse. Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. Testosterone abuse can lead to serious cardiovascular and psychiatric adverse reactions.
Suppression of spermatogenesis. Large doses of androgens, like JATENZO, can suppress spermatogenesis.
Hepatic adverse events. JATENZO is not known to cause liver adverse events; however, patients should be instructed to report any signs of hepatic dysfunction.
Retention of sodium and water.
Gynecomastia.
Sleep apnea. Testosterone may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung disease.
Changes in the serum lipid profile may require dose adjustment of lipid-lowering drugs or discontinuation of testosterone therapy.
Risk of hypercalcemia.
The most common adverse events of JATENZO (incidence ≥2%) are headache (5%), increased hematocrit (5%), hypertension (4%), decreased HDL (3%), and nausea (2%).
JATENZO can cause changes in insulin sensitivity or glycemic control and changes in anticoagulant activity. Use of testosterone and corticosteroids concurrently may increase fluid retention. Use of prescription and nonprescription analgesic cold medications with JATENZO have been known to increase blood pressure.
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Please see full Prescribing Information.
References
1. JATENZO® (testosterone undecanoate) capsules, for oral use CIII [package insert]. Fort Collins, CO: Tolmar, Inc.; 2025.
2. Data on file. Tolmar, Inc.; CLAR-15012 CSR.
3. Swerdloff RS, Wang C, White WB, et al. A new oral testosterone undecanoate formulation restores testosterone to normal concentrations in hypogonadal men. J Clin Endocrinol Metab. 2020;105(8):2515-2531.
4. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103:1715-1744.