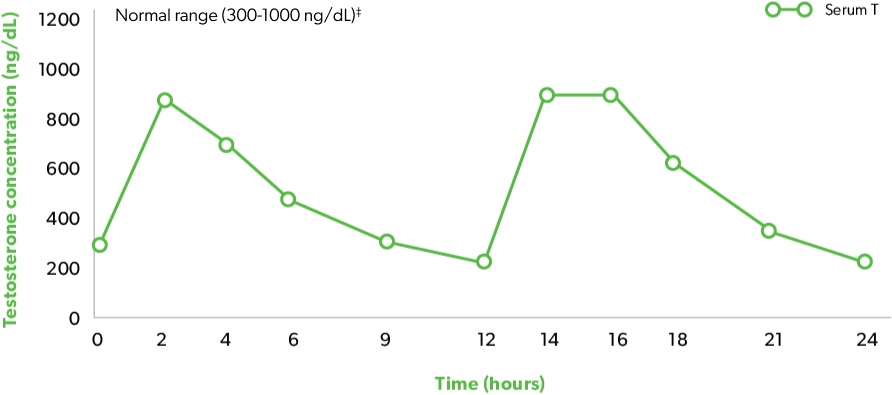
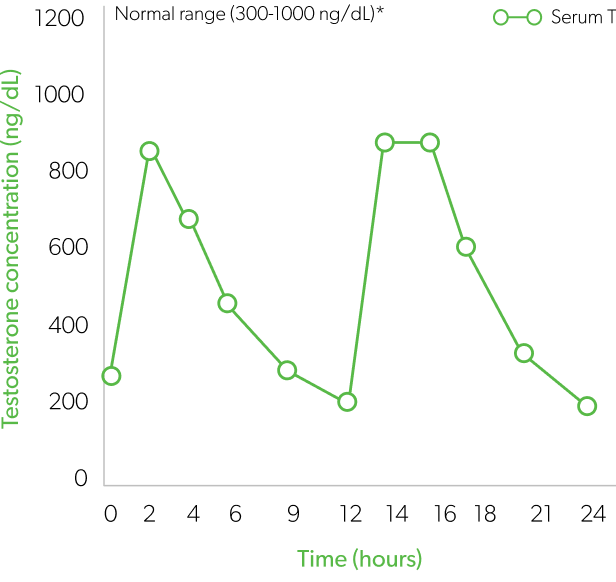
Indication and Limitations of Use:
JATENZO®(testosterone undecanoate) capsules, CIII, is an androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone:
- Primary hypogonadism (congenital or acquired)
- Hypogonadotropic hypogonadism (congenital or acquired)
Safety and efficacy of JATENZO in males less than 18 years old have not been established.
IMPORTANT SAFETY INFORMATION FOR JATENZO (testosterone undecanoate)
WARNING: INCREASES IN BLOOD PRESSURE
- JATENZO can cause blood pressure (BP) increases that can increase the risk of major adverse cardiovascular events (MACE), including non-fatal myocardial infarction, non-fatal stroke and cardiovascular death.
- Before initiating JATENZO, consider the patient’s baseline cardiovascular risk and ensure blood pressure is adequately controlled.
- Periodically monitor for and treat new-onset hypertension or exacerbations of pre-existing hypertension and re-evaluate whether the benefits of JATENZO outweigh its risks in patients who develop cardiovascular risk factors or cardiovascular disease on treatment.
- Due to this risk, use JATENZO only for the treatment of men with hypogonadal conditions associated with structural or genetic etiologies.
CONTRAINDICATIONS
JATENZO is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, in women who are pregnant, in men with a known hypersensitivity to JATENZO or its ingredients, or in men with hypogonadal conditions that are not associated with structural or genetic etiologies.
WARNINGS AND PRECAUTIONS
Increase blood pressure and Major Adverse Cardiovascular Events. JATENZO can increase blood pressure, which can increase the risk of MACE, with greater risk in patients with established cardiovascular disease or risk factors for cardiovascular disease.
Increase in hematocrit and polycythemia. High red blood cell counts increase the risk of clots, strokes, and heart attacks.
Benign prostatic hyperplasia (BPH). Patients may see worsening signs and symptoms of BPH.
Prostate cancer. Patients treated with androgens may be at increased risk for prostate cancer.
Venous thromboembolic events (VTE). Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients using testosterone replacement products like JATENZO.
Abuse. Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. Testosterone abuse can lead to serious cardiovascular and psychiatric adverse reactions.
Suppression of spermatogenesis. Large doses of androgens, like JATENZO, can suppress spermatogenesis.
Hepatic adverse events. JATENZO is not known to cause liver adverse events; however, patients should be instructed to report any signs of hepatic dysfunction.
Retention of sodium and water.
Gynecomastia.
Sleep apnea. Testosterone may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung disease.
Changes in the serum lipid profile may require dose adjustment of lipid-lowering drugs or discontinuation of testosterone therapy.
Risk of hypercalcemia.
ADVERSE EVENTS
The most common adverse events of JATENZO (incidence ≥2%) are headache (5%), increased hematocrit (5%), hypertension (4%), decreased HDL (3%), and nausea (2%).
DRUG INTERACTIONS
JATENZO can cause changes in insulin sensitivity or glycemic control and changes in anticoagulant activity. Use of testosterone and corticosteroids concurrently may increase fluid retention. Use of prescription and nonprescription analgesic cold medications with JATENZO have been known to increase blood pressure.
Please see full Prescribing Information, including BOXED WARNING on increases in blood pressure.
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.